MDR Medical Devices Regulation (EU 2017/745)
Corresponds to the current interpretation, including the current postponement according to EU 2023/607
This information has been drawn up by Beurer GmbH. We assume no liability for the accuracy, completeness or validity of the information.
We shall not be held liable for any form of damage resulting from the use of the information provided, unless intentional or grossly negligent conduct is proven against us.
What is the MDR?
As from 26 May 2021 it will be mandatory to apply the new EU Medical Devices Regulation (MDR; EU 2017/745), which replaces the existing Medical Device Directive (MDD; 93/42/EEC).
The MDR governs the approval of medical devices such as blood pressure monitors and clinical thermometers, and brings EU legislation into line with technical advances and changes in medical science in terms of law-making.
This has resulted in different, expanded rules and obligations for everyone involved in the supply chain. These rules and obligations must be considered and implemented independently by the parties concerned.
We have therefore published this document to inform you of what this means for your collaboration with Beurer and for you as a distributor of our devices.
Beurer shall ensure that our medical devices are switched over from the MDD to the MDR in good time. You can therefore continue ordering devices from Beurer without hesitation.
To avoid market disruption and to allow a smooth transition from the Directive to the Regulations, several transitional provisions are in place.
Deadlines for implementing the MDR

NOTE: All devices that were placed on the market before 31 December 2028 (e.g. in stock at distributors) may be resold without any further deadlines applying.
Blood glucose monitors, test strips and control solutions fall under Annex II List B (IVDD = In Vitro Diagnostic Directive) / Annex VIII Class C (IVDR = In Vitro Diagnostic Regulation).
The IVDR entered into force on 25 May 2017 at the same time as the MDR and will apply as from 26 May 2022.
* Postponement of the date of application by one year due to COVID-19 pandemic decided by the European Parliament on 17 April 2020.
Corresponds to the current interpretation, including the current postponement according to EU 2023/607
Who is who?
As a Beurer customer, you always have the role of "distributor". We would like to take this opportunity to inform you of the additional tasks and obligations that you will now need to comply with under the MDR.
If the distributor sells the device to a reseller, the distributor must also provide the reseller with the CE declaration. The reseller is therefore part of the supply chain and is subject to the same obligations as a distributor.

Overview of obligations for distributors
Distributors must make sure, by representative sampling, that the distributed medical devices are compliant with the obligations described in Art. 14 of the Medical Devices Regulation.
Inspection of device and documentation (according to MDR Art. 14 (2))
- The device has been CE marked.
- The EU Declaration of Conformity is available. If the Declaration of Conformity is missing, it is available for download free of charge from the Beurer website.
- Labels and instructions for use have been provided in the official languages of the Member States in which the device is made available (or in languages accepted by those Member States).
- The manufacturer's name has been indicated on the device or in the accompanying documentation.
- NEW! Where applicable, the device has a UDI (during certain transition periods the device may not necessarily have a UDI but is nevertheless classed as a medical device; see the explanation provided under "Unique Device Identifier – UDI").
Assurance of the storage and transport conditions recommended by the manufacturer (according to MDR Art. 14 (3))
- As was already the case for medical devices under the MDD, the storage and transport conditions have been stated on the decorative box and in the instructions for use and must be complied with.
Reporting procedure & documentation (according to MDR Art. 14 (2) – (6))
- If a device is non-compliant with the respective Regulation, then it is prohibited to sell the device.
- Should there be a reason to suspect that a device deviates from this Regulation, the distributor must inform the manufacturer immediately (see the service addresses).
- Distributors must inform the authorities and other economic operators (manufacturers and other distributors in the supply chain) if they suspect that a device has been falsified or that there is a serious risk to health from the device.
- NEW! Distributors must keep a register of complaints, non-conforming devices, recalls and withdrawals.
- Distributors must cooperate with the authorities and make available all the information, documentation and device samples they have at their disposal.
- Distributors must immediately report to the manufacturer any complaints and reports of suspected incidents related to a device that was made available.
The current reporting addresses for Beurer and Sanitas devices can be found on page 11 in the "Downloads and reporting addresses" section
Documentation of the supply chain
- from whom was the device purchased and to whom was it handed?
- the distributor's obligation to furnish proof ends when the device is handed over to a patient or a private end customer (MDR Art. 10 (8)).
Unique Device Identification
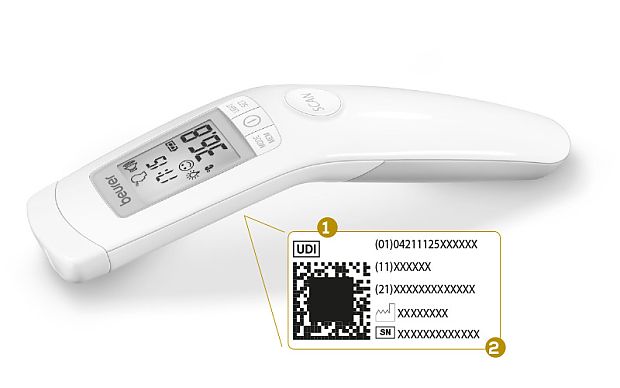
UDI stands for Unique Device Identifier. The MDR introduced the new requirement that, in future, all medical devices must feature this code.
Deadlines for applying the UDI to devices according to the MDR:
- Class III: by 26 May 2021
- Class IIa and IIb: by 26 May 2023
- Class I: by 26 May 2025
- (Reusable medical devices on which the UDI carrier must be applied directly to the device: 2 years later in each case, i.e. 26 May 2023, 2025 or 2027)
Additional notice: The UDI does not have to be applied to MDD devices
The UDI system will help to improve the identification and traceability of medical devices. All information is stated both in plain-text form (letters and numbers) and in machine-readable form (a barcode or a matrix code).
The purpose of the UDI is to make it possible to individually recall a device in the future. For distributors, this is only relevant in the event of a recall.
The MDR requires all medical devices to feature the UDI code*
- UDI code as a matrix code and as plain text
- Serial number
*The image shown is for illustrative purposes only – the actual depiction may vary.
How can I identify a medical device?
1.0 List of medical devices
Is the device included in the list of Beurer medical devices (see page 9)?
2.0 Type plate
The device is identifiable as a medical device if points 2.1 and 2.2. apply
2.1 CE marking

2.2 The "MD" symbol
If the "MD" symbol is included on the type plate, the device is a medical device that complies with the MDR.

3.0 Instructions for use
If the following statement is included in the instructions for use, the device is a compliant medical device:
"This device meets the requirements of the EU Directive 93/42/EEC concerning medical devices, as well as those of the Medizinproduktegesetz (German Medical Devices Act), and [...]"
or
"The device complies with the Regulation (EU) 2017/745 of the European Parliament and of the Council on medical devices, as well as the respective national provisions [...]"
Which devices from Beurer/ Sanitas are classed as medical devices?

FAQs
Which Beurer devices are affected by the MDR and what are the key deadlines?
Depending on the duration of use, degree of invasiveness and the extent to which the device can be reused, medical devices are categorised into four classes: I, IIa, IIb and III.
Classes Is, Im, Ir, IIa, IIb and III need to be assessed and certified by an appointed body (known as a "Notified Body"). Certificates issued in accordance with the previous MDD continue to apply in line with their specified term or separate regulations/extensions by the Notified Bodies.
Class I devices have so far not required a certificate. They will need to be compliant with the MDR as from 26 May 2021.
As from May 2021 will I still receive all the devices that I previously purchased?
For manufacturers, obtaining the necessary certification for medical devices according to the MDR entails a great deal of time and considerable expense. As a result of this high outlay in terms of time and cost, distributors may find that familiar devices are no longer available.
Beurer has switched over all Class I devices to the MDR in good time.
All selected devices from a higher class will have been switched over to the MDR in good time, by 31 December 2028 at the latest.
Medical devices in accordance with the MDD, with a valid MDD certificate or separate regulations/extensions by the Notified Body, may then be placed on the market until 31 December 2028 at the latest.
We would be glad to help you if you have any further questions about the MDR. Please don't hesitate to contact us at mdr-info@beurer.de
As a distributor, what exactly do I need to document under the MDR?
For a minimum period of 10 years (MDR Art. 10, Section 8), you must document how your devices have been distributed:
- From whom did I purchase the medical device?
- To which other economic operators was the medical device handed over?
NOTE:
The aforementioned obligation to furnish proof ends when the device is handed over to the patient or a private consumer
- If the device is sold in a pharmacy, the pharmacy is not required to record or save the details of the end customer.
The MDR also stipulates this documentation when handing over the device to health institutions (e.g. clinics, doctors' practices or nursing homes). However, at the present time this has not yet been definitively clarified (Art. 25, Section 2c).
Where can I find more information about the MDR?
More information is available on the following EU website entitled "Getting ready for the new regulations":
https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework/getting-ready-new-regulations
The following section is aimed specifically at distributors and importers:
https://ec.europa.eu/growth/sectors/medical-devices/getting-ready-new-regulations/authorised-representatives-importers-and_en
European database on medical devices – EUDAMED
- Central platform for all information on manufacturers, devices, certifications and clinical trials
- Access rights t.b.d.
- Will be published and updated gradually
- https://ec.europa.eu/growth/sectors/medical-devices/new-regulations/eudamed_en
- https://ec.europa.eu/tools/eudamed/#/screen/home
Downloads and reporting addresses for Beurer and Sanitas
CE Declarations of Conformity for Beurer devices:
https://www.beurer.com/mdr
CE Declarations of Conformity for Sanitas devices:
http://sanitas-online.de/web/de/landingpages/cedeclarationofconformity.php
Current reporting address for Beurer devices:
You can find the latest reporting addresses in your country for Beurer devices at: www.beurer.com –> Service –> Service addresses.
https://www.beurer.com/web/de/service/internationale-serviceadressen.php
Current reporting address for Sanitas devices:
E-Mail: service@sanitas-online.de
As from 26 May 2021 it will be mandatory to apply the new EU Medical Devices Regulation (MDR). The MDR governs the approval of medical devices and brings EU legislation into line with technical advances and changes in medical science in terms of law-making. We have therefore published this document to inform you of what this means for your collaboration with Beurer and for you as a distributor of our devices.
